“Each return of a controlled substance is more than a transaction – it’s a test of compliance, efficiency, and integrity.”
The moment for change: the compliance crossroads in pharmaceutical reverse logistics
Over 50% of DEA registrants still rely on paper DEA Form 222, despite its growing inefficiencies and rising compliance risks. As the Drug Enforcement Administration (DEA) has been making a push towards a modernized and electronic system for ordering controlled substances, particularly with the phasing out of paper DEA Form 222s, pharmaceutical reverse logistics providers – particularly 3PLs – are at a crossroads.
In the high-stakes world of pharmaceutical supply chains, reverse logistics providers (3PLs) walk a tightrope. On one side, patient safety, public trust, and brand reputation. On the other, increasingly complex DEA compliance requirements around handling Schedule I and II substances.
Every return or disposal involving a controlled substance is governed by stringent Drug Enforcement Administration (DEA) diversion control rules. Failing to meet these regulations isn’t just risky – it’s costly. With fines soaring into the millions and operational licenses at stake, 3PLs must act swiftly and smartly to modernize their processes.
Without digital transformation, many could face fines, operational disruption, and reputational damage. Now is the time to modernize.
Understanding the DEA Diversion Control regulation for 3PLs
Reverse logistics providers that manage returns of Schedule I and II controlled substances are classified as reverse distributors by the DEA. As outlined in 21 CFR 1300, they are subject to strict rules:
- Must be registered with the DEA as a reverse distributor
- Can only accept Schedule I/II substances with a valid DEA Form 222 or CSOS equivalent
- Required to validate sending/receiving party DEA registrations
- Must maintain complete, auditable records for at least two years
- Must report destruction events or transfer activity to the DEA promptly
These regulations protect the integrity of the pharmaceutical supply chain and help prevent diversion of controlled substances.
High-level reverse logistics workflow

Traditional (paper DEA 222) workflow
- Customer (distributor/health system/pharmacy) initiates the return and requests DEA Form 222
- 3PLs/Reverse Logistic provider prepares DEA Form 222 in triplicate (reverse distributor is the “purchaser”, the customer is the “supplier”), sends Copy 1 and Copy 2 to the customer, and retains Copy 3 for their own DEA records.
- Once the customer receives Form 222, they validate it, keep Copy 1, and ship the Schedule I/II substances with Copy 2 to the reverse distributor.
- Reverse distributor logs receipt, updates Copy 3 with received quantities and dates, and sends Copy 2 to the DEA (within 2 business days of product receipt).
- Begins process of segregation and destruction or return to manufacturer, depending on drug type and manufacturer policy.
- If drugs are destroyed, DEA Form 41 is used. If returned to the manufacturer (e.g., for credit), a new DEA Form 222 or CSOS order may be needed from the manufacturer to reverse the distributor.
- The reverse distributor then validates the received Form 222 before shipping the goods to the manufacturer.
Impact analysis: what’s at risk
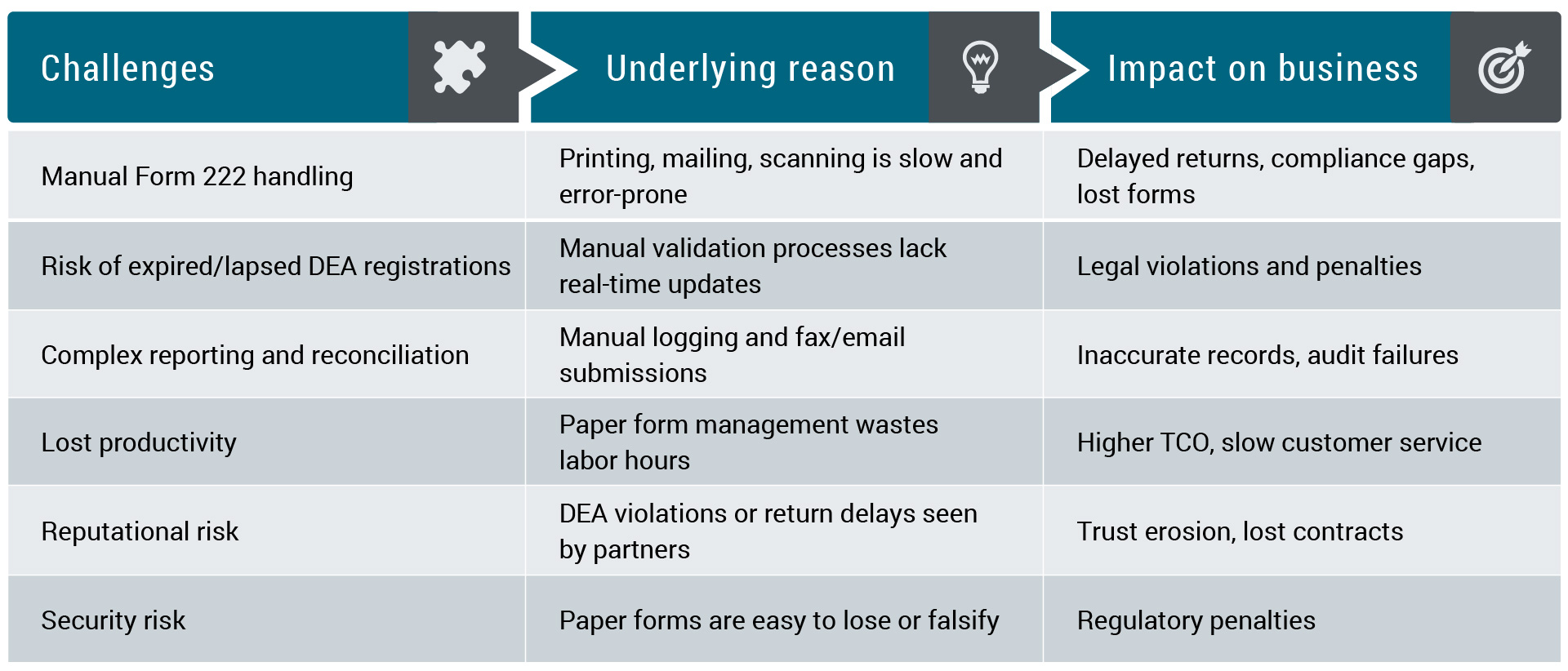
A call for modernization
The time for change is now. Reverse logistics is uniquely positioned to benefit from digitizing the DEA Form 222:
- Paper is being phased out: The DEA is encouraging providers to order controlled substances through CSOS.
- Audits are intensifying: Manual processes make it harder to defend against DEA audits.
- Digital creates value: CSOS is not just about compliance—it drives speed, accuracy, and cost-efficiency.
Axway CSOS powers the future of compliance reverse logistics
Axway offers a DEA-certified Controlled Substance Ordering System (CSOS) platform that empowers 3PLs and reverse logistics providers to:
- Digitally authorize Schedule I & II transfers with secure electronic 222 forms
- Automate certificate validation and partner registration with real-time DEA checks
- Ensure first-time-right documentation for every transaction
- Streamline returns, destruction, and reporting workflows
- Maintain full traceability and audit readiness
- Reduce return cycle time by 30-50% and lower operational costs
- Secure every transaction, ensuring compliance and trust
With Axway, 3PLs aren’t just digitizing forms. They’re digitizing trust.
Leaders choose Axway CSOS
Whether you’re focused on compliance, operational efficiency, or cost savings – Axway CSOS delivers measurable results:
- Executives: Lower TCO by eliminating paper, reducing fines, and streamlining reporting
- IT/Compliance: Centralize DEA recordkeeping with full audit trails and traceability
- Operations Leaders: Improve First Time Right, reduce cycle times, and increase customer satisfaction
A safer, smarter future starts here
Compliance doesn’t need to be cumbersome. With Axway CSOS, 3PLs and reverse logistics organizations can stay ahead of the DEA’s digital shift, reduce risk, and protect their brand.
Stay DEA-compliant and reduce costs with a modern API-enabled solution.


